Investigation of used Li-battery by field emission electron microscope (FE-SEM)
Since our company sees great potential in developing novel electrode materials for future batteries, the necessity of acquiring a solid knowledge on the ever-growing actual Li-battery technologies became obvious. As an important step, we carried out a comprehensive SEM and EDS investigation on both cathode and anode materials of a used cell phone battery that was completely discharged before dismantling. The exact compositions of these parts were not known, therefore all conclusions drawn in this regard are based strongly on literature data and, naturally, on our EDS elemental analysis results.
Cathode
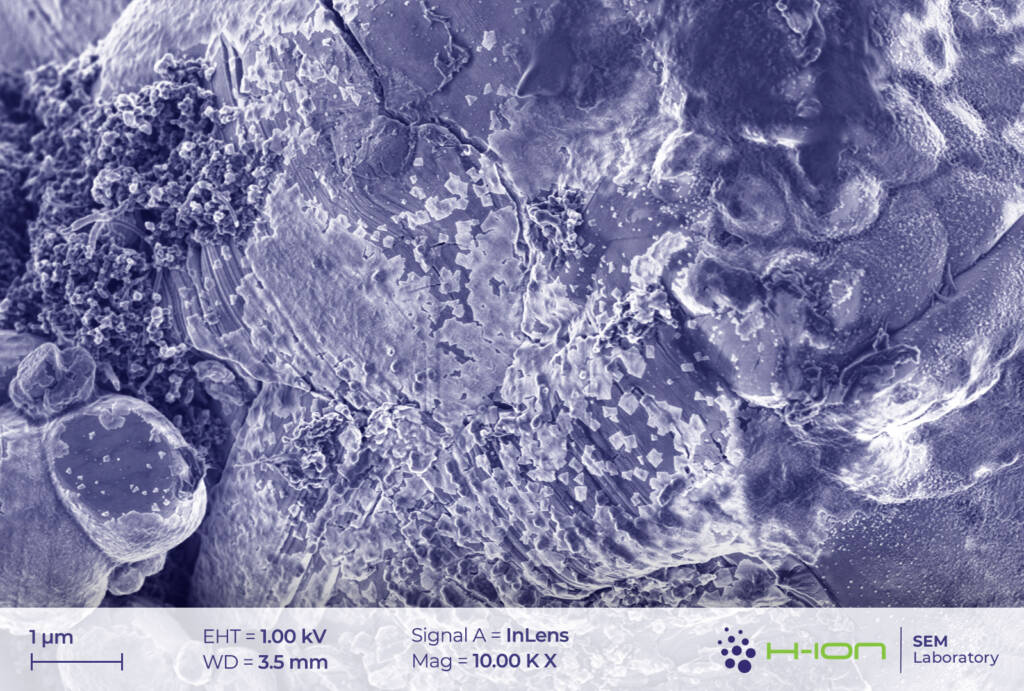
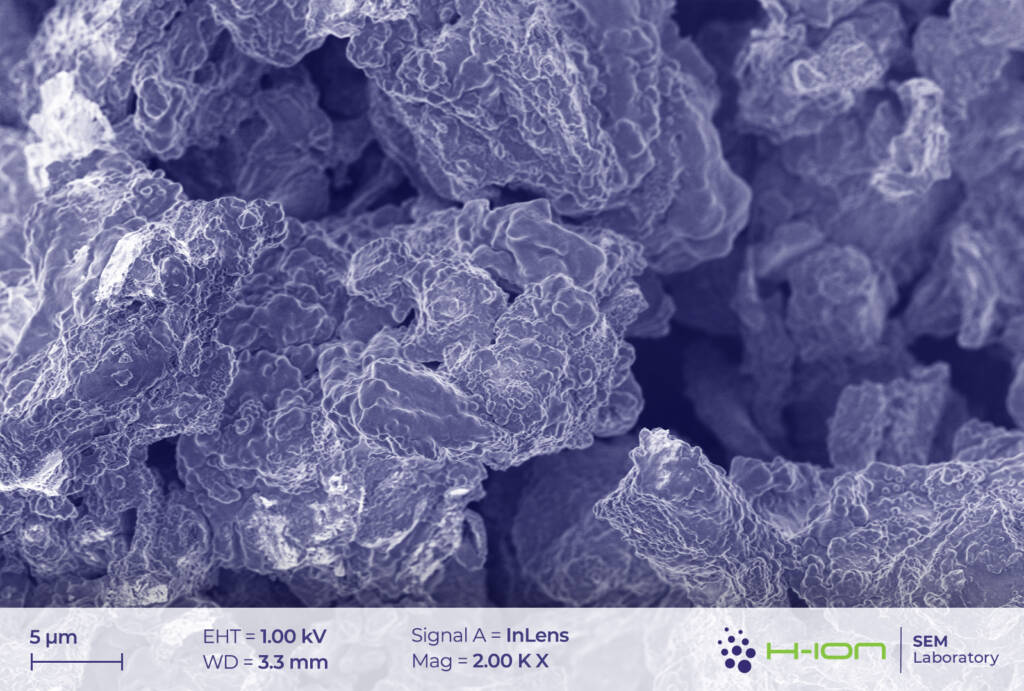
The main ingredient of the cathode’s active material was measured to be LiCoO2. Very small amounts of Mn and Ni (1.5-2.0% of the Co-content in at% for both) were also detected in some cases. This may suggest the existence of a blended cathode [1] rather than of a conventional NMC (Ni-Mn-Co) type, in which all three transition metals are present in comparable quantities and with Co having one of the lowest amongst all (e.g., NMC811, NMC532, NMC622). LiCoO2 grains exhibited multimodal size distribution (Figure 1.). The diameters of smaller crystallites were ranging from a few hundred nanometers to several microns, while the biggest ones had sizes around 10-20 mm.
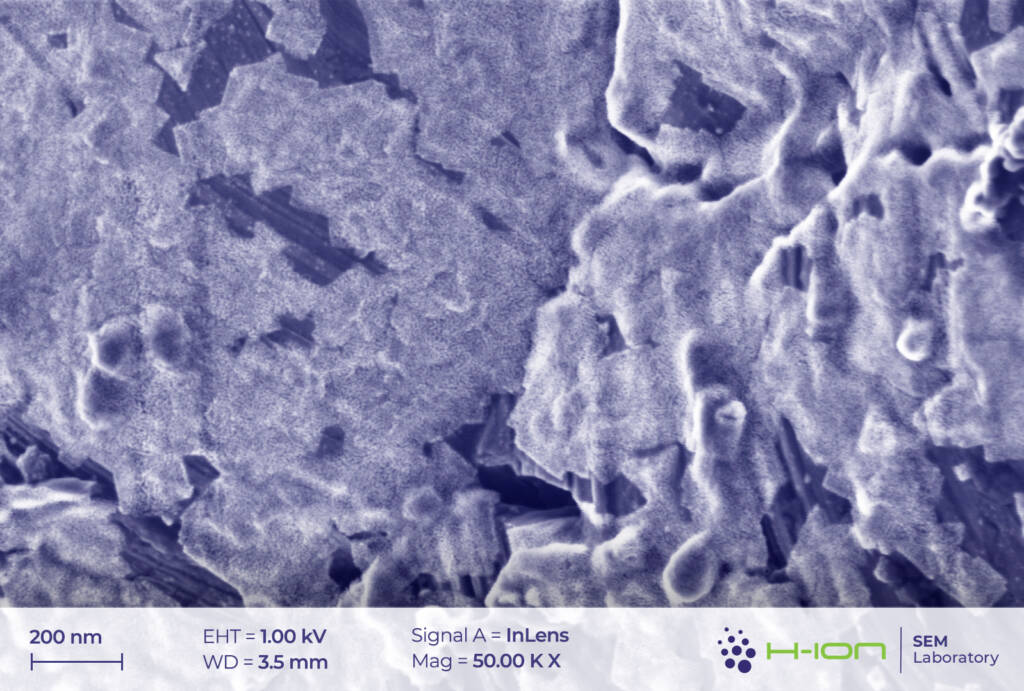
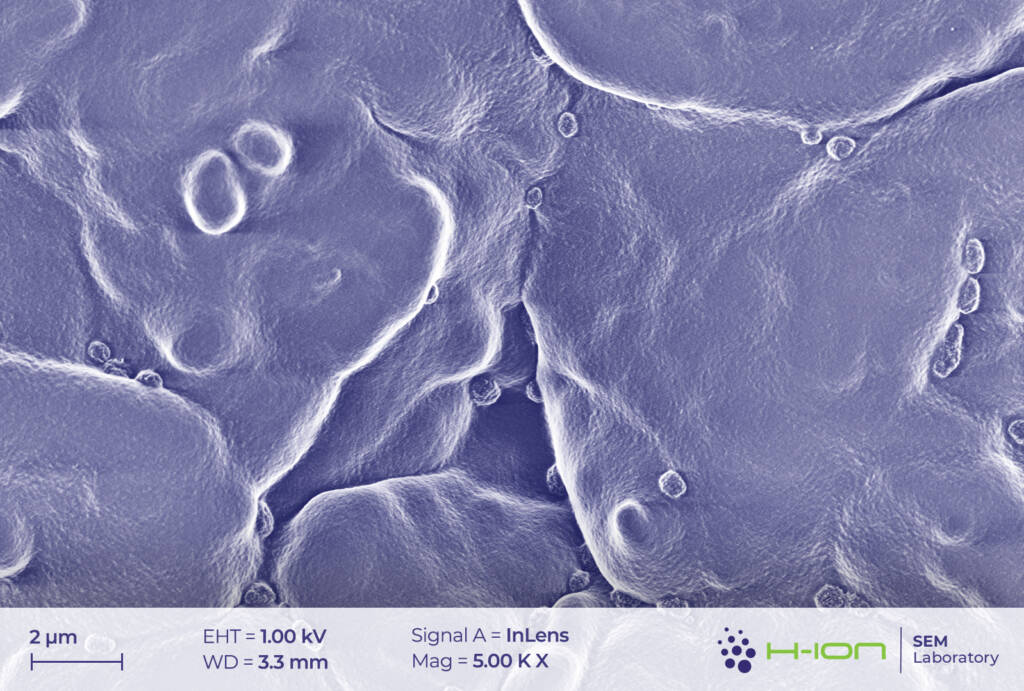
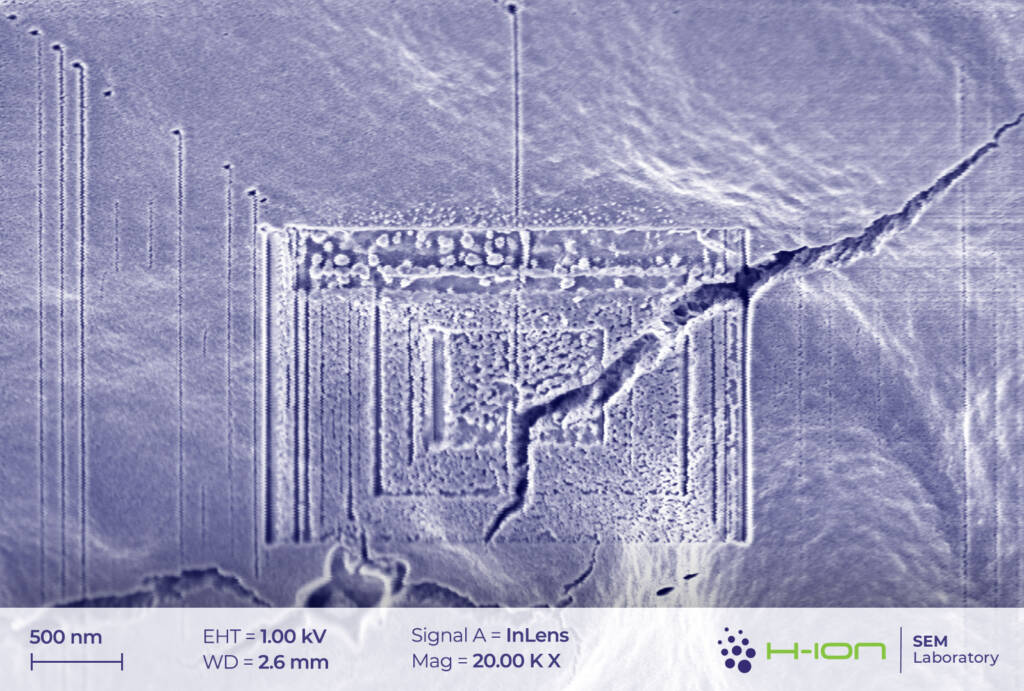
There is a bright-hued layer covering the majority of the particles which was identified as the so-called CEI (cathode electrolyte interphase). It is formed when dissolved metal ions (M) react with the electrolyte forming mostly M-fluorides, M-carbonates, M-hydroxides and these species are then deposited back onto the electrode surface [2]. At the perimeter of the continuous layer, some individual triangular and rectangular CEI islands appear (Figure 2 and/or 3.). The CEI film is vulnerable to the electron beam even at low acceleration voltage. Its structure becomes fuzzy upon a longer exposure (Figure 4).
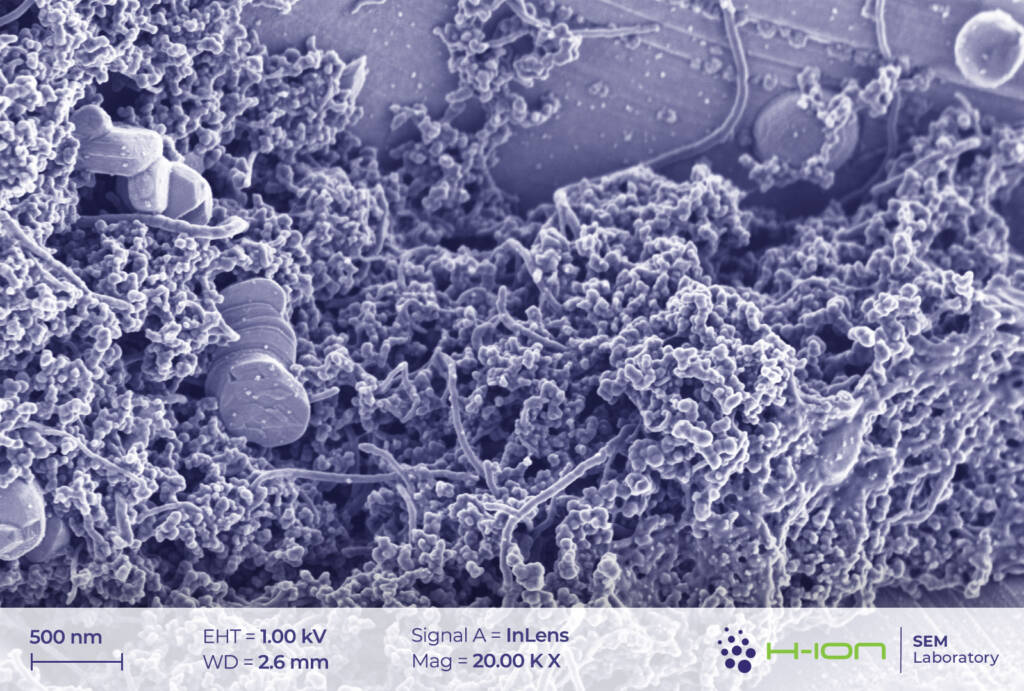
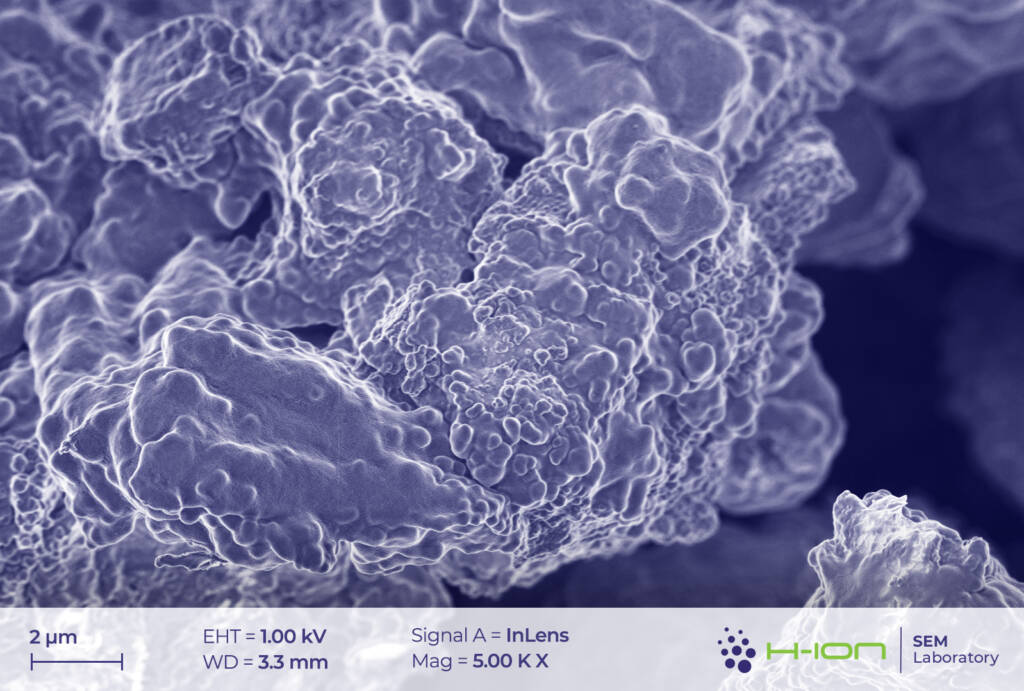
The network of conductive additives and the PVDF (polyvinylidene fluoride) binder is also visible in the micrographs (Figure 5). In our current case, the former set of materials comprises, most probably, carbon black and carbon nanotubes. The latter was reported to greatly improve the performance of the batteries owing to the advantageous electrical and mechanical properties [3]. PVDF, on the other hand, is sometimes imaged as filaments stretching between the grains (Figure 6).
Cathode particles crumble upon usage. Due to the countless contraction-expansion cycles occurring when charging-discharging and to the thermomechanical load acting on the system, the grains start to fragment [3] (Figure 2 and 7).
Anode
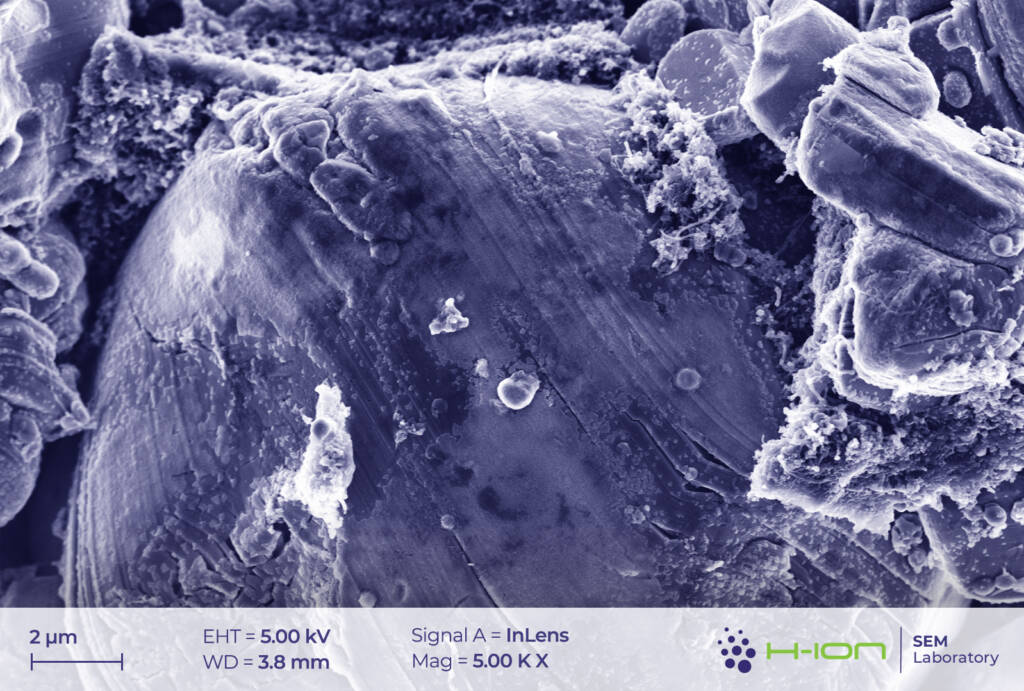
The anode is made up of graphite and the PVDF binder. Similarly to cathode’s case, a layer is built up from the decomposed electrolyte on the electrode surface, called SEI (solid electrolyte interphase) this time. However, the SEI is somewhat thicker (Figure 8-9) than the CEI and composed mainly of lithium fluoride (LiF), lithium carbonate (Li2CO3), lithium methyl carbonate (LiOCO2CH3), lithium ethylene dicarbonate (LiOCO2CH2)2 and lithium oxide (Li2O) [2].
At a location where the SEI layer was exfoliated from the electrode, cleaner but presumably still non-pristine graphite particles have emerged (Figure 10-11). Additionally, like it was experienced for the CEI, the SEI can also be damaged by beam even at low electron energy (Figure 12).
References
[1] Heubner C., Liebmann T., Lämmel C., Schneider M., Michaelis A., J. Energy Storage, 2018, 20, 101–108.
[2] Edge J. S., O’Kane S., Prosser R., Kirkaldy N. D., Patel A. N., Hales A., Ghosh A., Ai W., Chen J., Yang J., Li S., Pang M.-C., Bravo Diaz L., Tomaszewska A., Marzook M. W., Radhakrishnan K. N., Wang H., Patel Y., Wubd B., Offer G. J., Phys. Chem. Chem. Phys., 2021, 23, 8200–8221.
[3] Sheem K., Lee Y. H., Lim H. S., J. Power Sources, 2006, 158, 1425—1430.